Tailpipe Filter Traps CO2
This seems like a simple idea and as is always the case will the benefits outweigh the costs? But can we afford to not do everything we can, no what the cost to protect our environment?
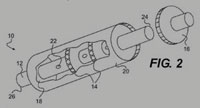
The flow-thru filter is easily attached to the tailpipe of the vehicle. The filter matrix is treated with a basic chemical compound. The vehicle exhaust is then diverted into the carbon-capture filter, which traps CO2 in a flow-by chemical reaction. The filter matrix acts as a carbon sink, capturing harmful CO2. Once the filter is saturated with carbon, after approximately 3500 miles, it can be easily removed from the device and exchanged with a new filter.
The captured CO2 from the saturated filter is water-soluble and can then be safely converted into a useful industrial solid. This process provides a safe method of carbon storage.
The CO2 filter is positioned within the stainless steel housing to absorb the engine CO2 waste content by impulse collisions within the filter media. The impulse is equal to the change in momentum at points along the length of the filter. The impulse advantage is the product of the force of exhaust acting on the filter at impact points and the time the action takes place. The flow-by reaction in effect is a mild alkaline pH reacting with dilute acetic CO2 in the exhaust. Eventually the base solution becomes acetic when the filter is saturated with CO2.
Source: Vehicle exhaust goes into carbon-capture filter, which traps CO2
Every gallon of gasoline burned in a car’s engine produces CO2 and about 14% by volume is in the exhaust. Thirty to fifty percent of CO2 in the exhaust may be captured by a mix of potassium hydroxide and water dispersed within the ceramic wool support of a CO2 filter.
The captured CO2 changes the base media chemically into a mildly acidic composition – about the pH of a blueberry. The filter is then removed after experiencing high mileage and is soaked in a tank of water.
The CO2 dissolves in the water, and calcium hydroxide is added. The result is immediate. Calcium carbonate forms in the water due to the abundance of dissolved CO2 from the filter.
The amount CaCO3 formed in this manner directly correlates to the amount of CO2 absorbed from the car exhaust. This is calculated simply mole percent times mass.

Leave a Reply
Want to join the discussion?Feel free to contribute!